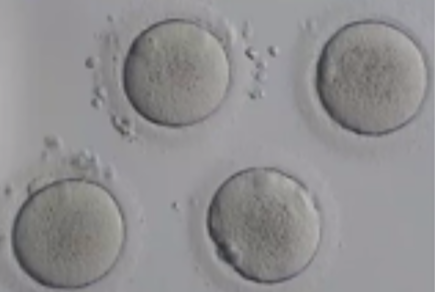
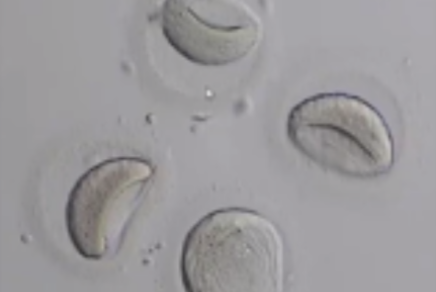
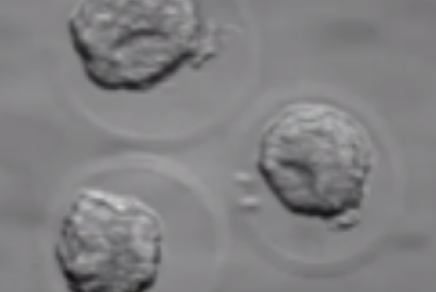
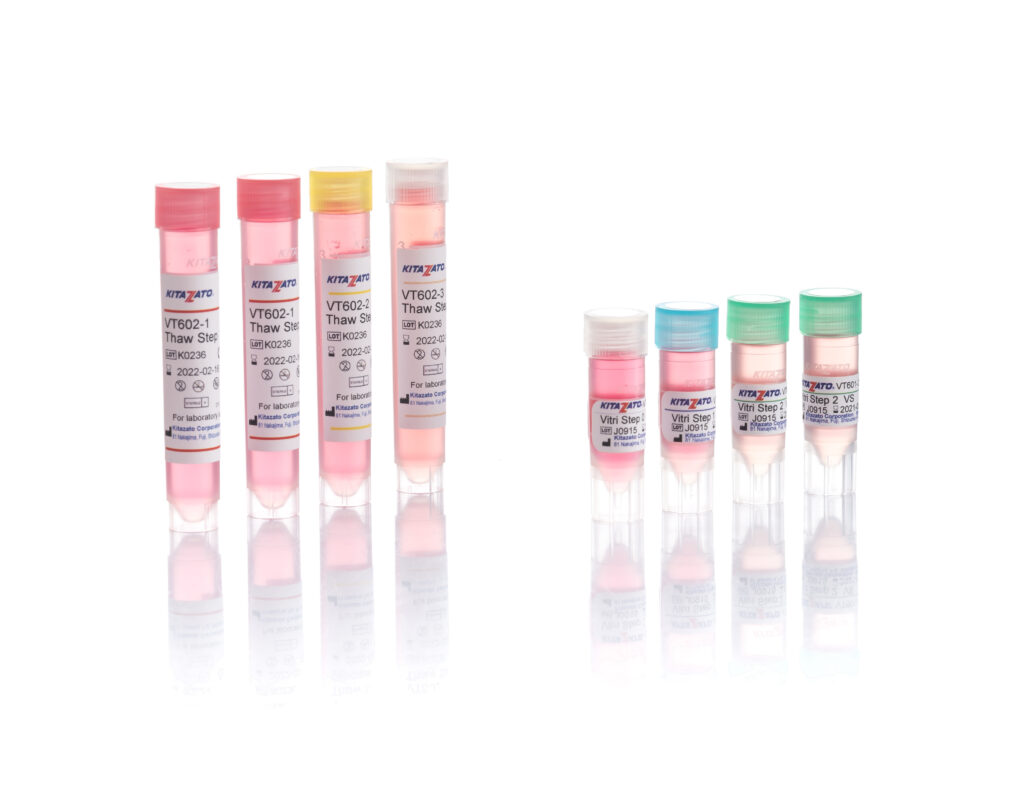
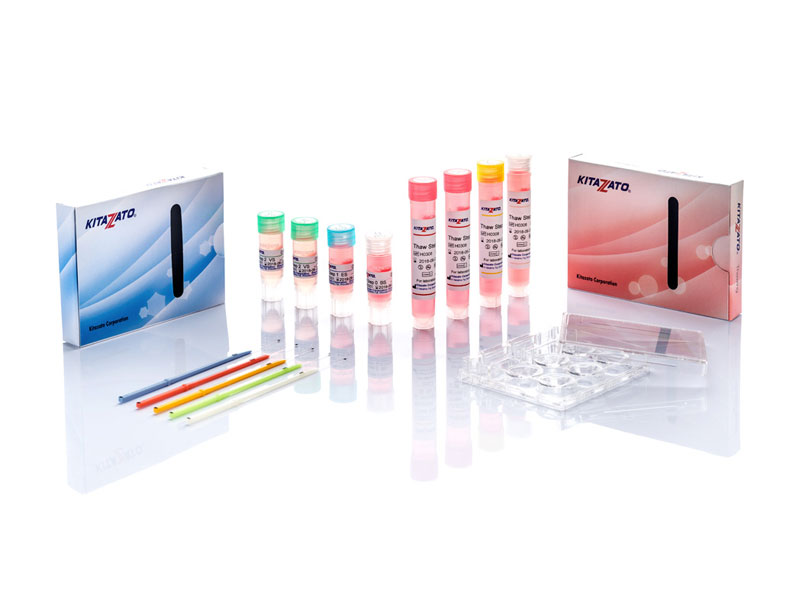
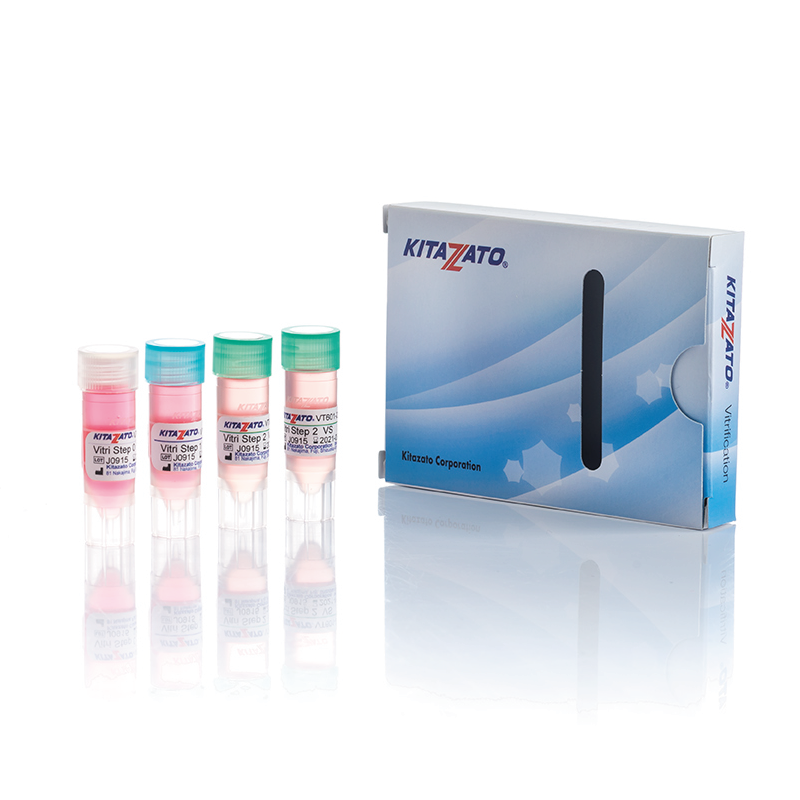
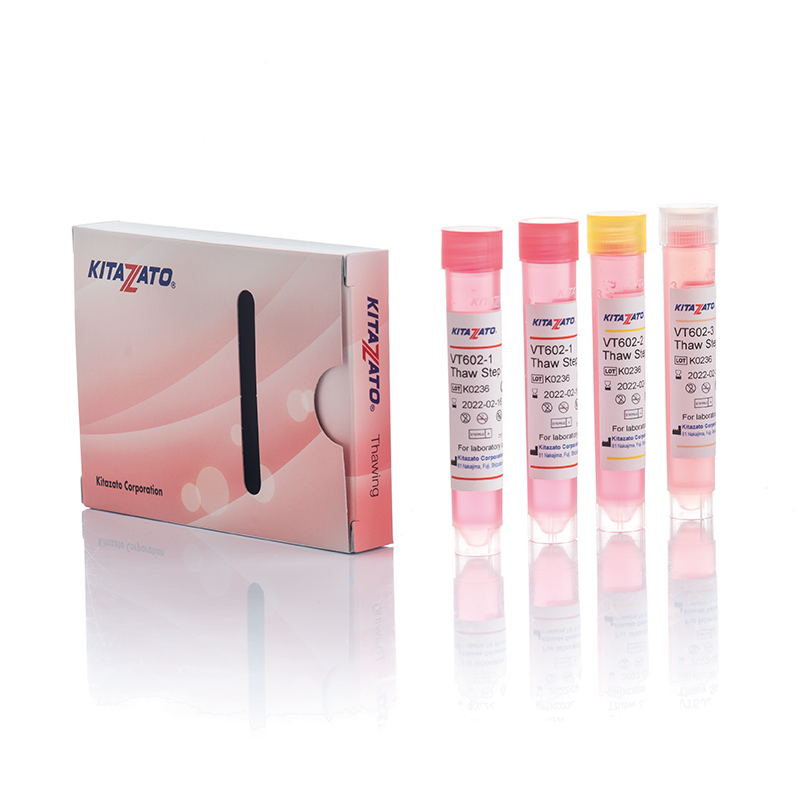
Before being plunged into LN, oocytes or embryos are exposed to a non-vitrifying CPA solution (ES – Equilibration Solution), containing only permeable CPAs that penetrate the cells, then to a vitrifying CPA solution (VS – Vitrification Solution), containing both permeable and non-permeable CPAs.
Upon exposure to the non-vitrifying CPAs, the cell immediately adjusts its osmolarity by losing water, and shrinks.
The CPAs then enter the cell more slowly, due to their low membrane permeability.
The time of exposure to the non-vitrifying solutions at a defined temperature is of critical importance, as it determines the intracellular concentration of CPA.
The vitrifying solution causes further cellular dehydration, which concentrates the intracellular CPA.
The overall objective is to create an intracellular environment more stable that will remain vitrified.